光化学降解木质素

Supporting Information
A Photochemical Strategy for Lignin Degradation at Room Temperature
John D. Nguyen, Bryan S. Matsuura, and Corey R. J. Stephenson
Department of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109, United States
Table of Contents
I. GENERAL INFORMATION .......................................................................................................... S2 – S3 II. GENERAL PROCEDURE A: BENZYLIC OXIDATION WITH [4-AcNH-TEMPO]BF4.................. S4 III. GENERAL PROCEDURE B: REDUCTIVE C–O BOND CLEAVAGE............................................ S4 IV. GENERAL PROCEDURE C: TWO-STEP DEGRADATION PROTOCOL ..................................... S4 V. OPTIMIZATION & CONTROL REACTIONS .................................................................................... S5 VI. SUBSTRATE SCOPE FOR OXIDATION WITH [4-AcNH-TEMPO]BF4 ........................................ S6 VII. INVESTIGATION OF VISIBLE LIGHT-MEDIATED BENZYLIC OXIDATION ......................... S7 VIII. COMPOUND INDEX ....................................................................................................................... S8 IX. COMPOUND CHARACTERIZATION .................................................................................... S9 – S20 X. BATCH TO FLOW REACTION ........................................................................................................ S21 XI. REDUCTIVE C–O BOND CLEAVAGE IN THE PRESENCE OF LIGNOSULFONATES........... S22 XII.PROPOSED MECHANISMS FOR THE FRAGMENTATION OF SUBSTRATE 8 ..................... S23 XIII. 1H NMR and 13C NMR Spectra ............................................................................................. S24 – S49
S1
S2
General information:
Chemicals were either used as received or purified according to the procedures outlined in Purification of Common Laboratory Chemicals C oven or flame dried under vacuum and cooled under inert atmosphere before use. Reactions were monitored by TLC and visualized by a dual short wave/long wave UV lamp and stained with an ethanolic solution of potassium permanganate, ceric ammonium molybdate, or anisaldehyde. Column flash chromatography was performed using 230-400 mesh silica gel. Yields refer to chromatographically and spectroscopically pure compounds, unless otherwise noted.
1
H- and 13C- NMR spectra were recorded using an internal deuterium lock on Varian
Mercury 300, Varian Unity Plus 400, or a Varian 500 spectrometers. All signals are reported in ppm with the internal reference of 7.26 ppm or 77.0 ppm for chloroform. Data are presented as follows: multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, quint = quintet, m = multiplet, br = broad, dd = doublet of doublet, dt = doublet of triplet, etc), coupling constant (J/Hz) and integration. Infrared spectra were recorded on a Perkin Elmer BX FT-IR. Absorptions are given in wavenumbers (cm -1). High resolution mass spectra were obtained on a Waters? Micromass ? AutoSpec Ultima ? with ESI high resolution mass spectrometer.
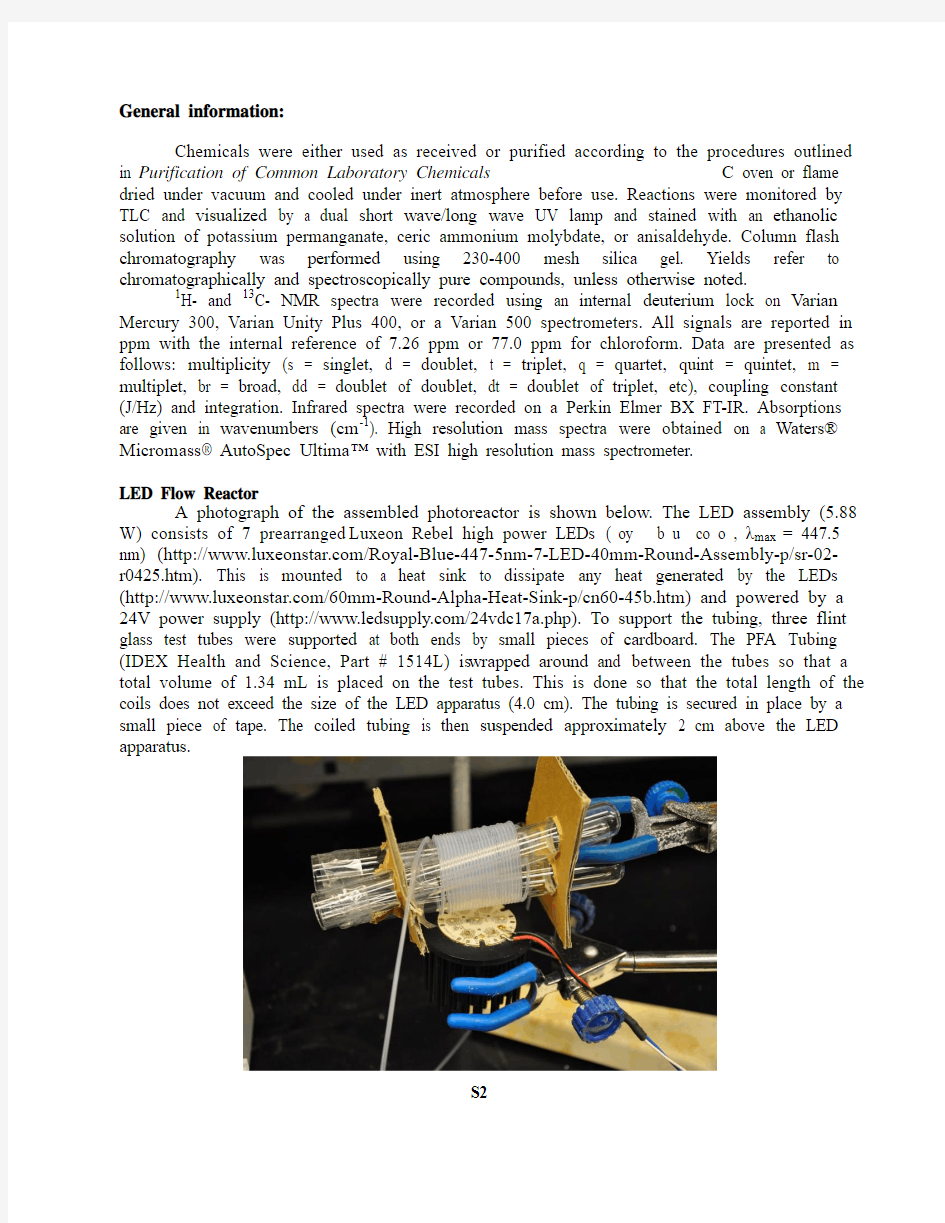
LED Flow Reactor
A photograph of the assembled photoreactor is shown below. The LED assembly (5.88 W) consists of 7 prearranged Luxeon Rebel high power LEDs ( oy b u co o , λmax = 447.5 nm) (https://www.wendangku.net/doc/002727762.html,/Royal-Blue-447-5nm-7-LED-40mm-Round-Assembly-p/sr-02-r0425.htm). This is mounted to a heat sink to dissipate any heat generated by the LEDs (https://www.wendangku.net/doc/002727762.html,/60mm-Round-Alpha-Heat-Sink-p/cn60-45b.htm) and powered by a 24V power supply (https://www.wendangku.net/doc/002727762.html,/24vdc17a.php). To support the tubing, three flint glass test tubes were supported at both ends by small pieces of cardboard. The PFA Tubing (IDEX Health and Science, Part # 1514L) is wrapped around and between the tubes so that a total volume of 1.34 mL is placed on the test tubes. This is done so that the total length of the coils does not exceed the size of the LED apparatus (4.0 cm). The tubing is secured in place by a small piece of tape. The coiled tubing is then suspended approximately 2 cm above the LED apparatus.
S3
The photoreactor tubing is the connected to the peristaltic pump tubing (IDEX Health and Science, Part # SC0717) by means of a conical adapter (IDEX Health and Science, Part # P-797) which contains the appropriate female nut, ferrule and washer. Likewise another short piece of PFA tubing, for delivery of the reaction mixture, was connected to the other end of the peristaltic pump tubing and fitted with a 20 gauge needle to pierce the septum of the reaction flask.
General Procedure A: Benzylic Oxidation with [4-AcNH-TEMPO]BF4. (SI Table 2)
A 5 dram vial with a magnetic stir bar was charged with the corresponding benzylic alcohol
(1.00 mmol, 1.00 equiv), dichloromethane (10.0 mL), silica gel (100 wt. % of benzylic alcohol), and [4-AcNH-TEMPO]BF4 (1.05 mmol, 1.05 equiv). The vial was capped and the heterogenous mixture was stirred at room temperature until it was complete (as judged by TLC analysis). The reaction mixture was vacuum filtered through a pad of silica on a glass-fritted funnel (pore size C) and an additional 30 mL of dichloromethane (10 mL portions) was used to rinse the product from the silica. The filtrate was concentrated in vacuo and the crude residue can be used directly in General Procedure B or purified by chromatography on silica gel to afford the desired product. General Procedure B: Reductive C–O Bond Cleavage. (Substrates 1-8)
A 5 dram vial with a magnetic stir bar was charged with the corresponding benzylic ketone (1.00 mmol, 1.00 equiv), MeCN (5.0 mL), N,N-diisopropylamine (3.0 mmol, 3.0 equiv), formic acid (3.0 mmol, 3.0 equiv) and [Ir(ppy)2(dtbbpy)]PF6(0.010 mmol, 0.010 equiv). The vial was capped and the reaction mixture was stirred at room temperature until it was complete (as judged by TLC analysis). The solvent was removed from the crude mixture in vacuo and was dissolved in EtOAc. The contents were poured into a separatory funnel containing 25 mL of EtOAc and 25 mL of deionized water. The layers were separated and the aqueous layer was extracted with EtOAc (2 × 25 mL). The combined organic layers were washed with brine, dried (Na2SO4), and concentrated in vacuo. The residue was purified by chromatography on silica gel to afford the desired products.
General Procedure C: Two-Step Degradation Protocol. (Substrates 9-11)
A 5 dram vial with a magnetic stir bar was charged with the corresponding benzylic alcohol
(1.00 mmol, 1.00 equiv), dichloromethane (10.0 mL), silica gel (100 wt. % of benzylic alcohol), and [4-AcNH-TEMPO]BF4 (1.05 mmol, 1.05 equiv). The vial was capped and the heterogenous mixture was stirred at room temperature until it was complete (as judged by TLC analysis). The reaction mixture was vacuum filtered through a pad of silica on a glass-fritted funnel (pore size C) and an additional 30 mL of dichloromethane (10 mL portions) was used to rinse the product from the silica. The filtrate was concentrated in vacuo and the crude residue was combined with MeCN (5.0 mL), N,N-diisopropylamine (3.0 mmol, 3.0 equiv), formic acid (3.0 mmol, 3.0 equiv) and [Ir(ppy)2(dtbbpy)]PF6 (0.010 mmol, 0.010 equiv) in a 5 dram vial. The vial was capped and the reaction mixture was stirred at room temperature until it was complete (as judged by TLC analysis). The solvent was removed from the crude mixture in vacuo and was dissolved in EtOAc. The contents were poured into a separatory funnel containing 25 mL of EtOAc and 25 mL of deionized water. The layers were separated and the aqueous layer was extracted with EtOAc (2 × 25 mL). The combined organic layers were washed with brine, dried (Na2SO4), and concentrated in vacuo. The residue was purified by chromatography on silica gel to afford the desired products.
S4
SI Table 1. Optimization of Reductive Cα-O Bond Cleavage & Control Reactions
S5
SI Table 2. Selective Benzylic Oxidation of Lignin Model Compounds With [4-AcNH-TEMPO]BF4
S6
SI Table 3. Investigation of Visible Light-Mediated Oxidation of Benzylic Alcohols
S7
Compound Index
S8
Compound Characterization
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanone (1):Substrate 1 was prepared according to a literature procedure.1 A round bottom flask equipped with a reflux condenser was charged with 2-bromo-1-(4-methoxyphenyl)ethanone (13.7 g, 60 mmol), potassium carbonate (12.3 g, 89 mmol), guaiacol (8.2 mL, 74 mmol), and acetone (250 mL). The resulting suspension was stirred and heated to reflux for 3 h, after which it was filtered through celite and concentrated in vacuo. The resulting solid purified by chromatography on SiO2(70:30, hexanes/EtOAc) afforded 1 (13.9 g, 51 mmol, 86%) as a colorless solid.
R f (EtOAc/hexane 1:3): 0.20;
IR (neat): 2936, 2837, 1689, 1597, 1501, 1212, 1168, 1127, 1023, 832, 739 cm-1;
1H NMR (CDCl
, 4 MHz): δ 8 3 (d, J = 8.4 Hz, 2H), 7.00–6.90 (m, 2H), 6.97 (d, J = 8.4 Hz,
3
2H), 6.88–6.84 (m, 2H), 5.29 (s, 2H), 3.89 (s, 6H);
13C NMR (CDCl
, 175 MHz): δ 193.1, 163.9, 149.7, 147.6, 130.5, 127.7, 122.3, 120.8, 114.7,
3
113.9, 112.2, 72.0, 55.9, 55.5;
HRMS (ESI) m/z calculated for C16H16O4+ ([M+1]+) 273.1121, found 273.1110.
2-(4-methoxyphenyl)-2-oxoethyl acetate (2):Substrate 2 was prepared according to literature procedure.2 A suspension of 2-bromo-1-(4-methoxyphenyl)ethanone (4.8 g, 21 mmol) in 22 mL of ethanol was prepared round-bottomed flask, and a solution of sodium acetate trihydrate (3.2 g, 24 mmol) in 11 mL of water and 1.1 mL of acetic acid was added. The mixture was heated at reflux for 2.5 h, then cooled to room temperature, and refrigerated overnight. In some cases, a solid separated that was collected by filtration and was found to be pure acetate. In other cases, most of the ethanol was removed under reduced pressure, and the resulting oily mixture was distributed between 30 mL EtOAc and 20 mL of a semisaturated, ice-cold NaHCO3 solution. The organic extracts were washed in sequence with 10 mL of semisaturated brine, dried with sodium
1J. Am. Chem. Soc.2010, 132, 12554.
2J. Med. Chem.2007, 50, 21.
S9
sulfate, and evaporated in vacuo. Purified by chromatography on SiO2 (70:30, hexanes/EtOAc) afforded 2 (4.0 g, 19 mmol, 90%) as a colorless solid. Spectral data are consistent with those reported in the literature.3
R f (EtOAc/hexanes 1:3): 0.20;
1H NMR (CDCl
, 4 MHz): δ 8 (d, J = 7.4 Hz, 2H), 6.83 (d, J = 7.3 Hz, 2H), 5.19 (s, 2H),
3
3.74 (s, 3H), 2.11 (s, 3H).
1-(2-methoxyphenoxy)propan-2-one (3):Substrate 3was prepared according to a literature procedure.4A mixture of guaiacol (0.62 g, 5.0 mmol), chloroacetone (0.69 g, 7.5 mmol) and K2CO3 (1.0 g, 7.5 mmol) in acetone (10 mL) was stirred at 60 °C for 3 hours. Then, K2CO3 was filtered and the solvent removed under reduced pressure and purification by chromatography on SiO2 (75:25, hexanes/EtOAc) afforded 3 (0.74 g, 4.1 mmol, 82%) as a clear and colorless oil.
R f (EtOAc/hexanes 1:3): 0.25;
IR (neat): 2918, 2837, 1717, 1592, 1501, 1250, 1125, 1024, 965, 740 cm-1;
1H NMR (CDCl
, 400 MHz): δ (dt, J = 8.2, 1.4 Hz, 1H), 6.94 (dd, J = 8.2, 1.8 Hz, 1H), 6.89
3
(dt, J = 8.0, 1.8 Hz, 1H), 6.79 (dd, J = 8.0, 1.4 Hz, 1H), 4.60 (s, 2H), 3.90 (s, 3H), 2.30 (s, 3H); 13C NMR (CDCl
, 175 MHz): δ 205.9, 149.4, 147.1, 122.3, 120.6, 114.1, 112.0, 74.1, 55.6, 26.2;
3
) 181.0859, found 181.0855.
HRMS (ESI) m/z calculated for C10H12O3+ ([M+1]+
3-hydroxy-2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)propan-1-one (5):Substrate 5was prepared according to a literature procedure.5 A solution of 1 (1.5 g, 5.5 mmol) in DMSO (30 mL) containing K2CO3(1.5 g, 11 mmol) was stirred for 30 min at room temperature before
3Chin. J. Chem.2010, 28, 294.
4Eur. J. Org. Chem.2012, 2012, 1499.
5J. Org. Chem.2010, 75, 6549.
S10
adding formaldehyde (37 %) (0.82 mL, 11 mmol). The resulting solution was stirred for 3 h and 2 N NaOH (15 mL) was added and stirred for 1 h. After addition of 1 N HCl, the solution was extracted with ethyl acetate. The extracts were dried, concentrated in vacuo, and purification by chromatography on SiO2(50:50, hexanes/EtOAc) afforded 5(0.83 g, 2.8 mmol, 50%) as a colorless solid.
R f (EtOAc/hexanes 3:2): 0.31;
IR (neat): 3474, 2942, 2840, 1684, 1599, 1504, 1254, 1173, 1128, 1026, 974, 840, 742 cm-1;
1H NMR (CDCl
, 400 MHz): δ 8 6 (d, J = 8.6 Hz, 2H), 7.02 (t, J = 7.6 Hz, 1H), 6.98–6.89 (m,
3
4H), 6.83 (t, J = 7.2 Hz, 1H), 5.40–5.35 (m, 1H), 4.07 (t, J = 5.1 Hz, 2H), 3.89 (s, 3H), 3.87 (s, 3H), 3.00 (t, J = 5.6 Hz, 1H);
13C NMR (CDCl
, 175 MHz): δ 195.0, 164.0, 150.5, 146.9, 131.3, 127.9, 123.6, 121.1, 118.6,
3
114.0, 112.3, 84.7, 63.6, 55.8, 55.5;
HRMS (ESI) m/z calculated for C17H18O5+ ([M+1]+) 303.1227, found 303.1225.
3-hydroxy-1-(4-methoxyphenyl)propan-1-one (table 1, entry 5, product 1):According to General Procedure B, 5 (0.30 g, 1.0 mmol), DIPEA (0.52 mL, 0.39 g, 3.0 mmol), formic acid (0.11 mL, 0.14 g, 3.0 mmol) and [Ir(ppy)2(dtbbpy)]PF6 (9.2 mg, 10 μmo ) M CN (5.0 mL) afforded S7 (0.15 g, 0.85 mmol, 85%) and guaiacol (0.11 g, 0.88 mmol, 88%) after purification by chromatography on SiO2(50:50, hexanes/EtOAc). Spectral data are consistent with those reported in the literature.6
R f (EtOAc/hexanes 3:2): 0.28;
1H NMR (CDCl
, 4 MHz): δ 7.93 (d, J = 9.0 Hz, 2H), 6.93 (d, J = 9.0 Hz, 2H), 4.00 (t, J = 5.5
3
= 5.5 Hz, 2H,), 2.74 (br s, 1H).
Hz, 2H), 3.89 (s, 3H), 3.16 (t, J
6Tetrahedron2010, 66, 3995.
S11
1-(3,4-dimethoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol (11):The synthesis of 11 was adapted from the literature procedure.7To o ut o of β-hydroxyester7(3.4 g, 9.0 mmol) in THF/H2O (5 mL, 3:1 ratio) was added sodium borohydride (1.7 g, 45 mmol) in portions. The reaction was allowed to stir at room temperature overnight. The reaction was diluted with ethyl acetate and washed with water and brine, then dried over sodium sulfate. The organic layer was then concentrated and purified by chromatography on SiO2 (60:40, hexanes/EtOAc) afforded 11 (2.7 g, 89%) as a 2.5:1 mixture of diastereomers. Spectral data are consistent with those reported in the literature.8
R f (hexanes/DCM/acetone/MeOH 6:2:1.5:0.5): 0.40;
1H NMR (CDCl
, 5 MHz): δ 7.14 (dd, J = 7.9, 1.5 Hz, 1H, minor diastereomer), 7.11-7.05 (m,
3
1H, major diastereomer, 1H minor diastereomer, overlap), 7.01-6.89 (m, 5H, major diastereomer, 2H, minor diastereomer, overlap), 6.87-6.83 (m, 1H, major diastereomer, 1H, minor diastereomer, overlap), 4.99 (d, J = 4.7 Hz, 1H, minor diastereomer), 4.98 (t, J = 4.7 Hz, 1H, major diastereomer), 4.16 (ddd, J= 6.0, 4.7, 3.4 Hz, 1H, major diastereomer), 4.04 (m, 1H, minor diastereomer), 3.95-3.89 (m, 1H, major diastereomer), 3.92 (s, 3H, minor diastereomer), 3.88 (s, 3H, major diastereomer, 3H, minor diastereomer), 3.88 (s, 3H, major diastereomer, 3H minor diastereomer), 3.69 (m, 1H, minor diastereomer) 3.66 (m, 1H, major diastereomer), 3.63 (m, 1H minor diastereomer), 3.48 (dd, J
= 12.5, 3.9 Hz, 1H, minor diastereomer).
1-(3,4-dimethoxyphenyl)-3-hydroxy-2-(2-methoxyphenoxy)propan-1-one (6): According to General Procedure A, 11 (334 mg, 1.0 mmol), [4-AcNH-TEMPO]BF4 (315 mg, 1.1 mmol), and silica (334 mg) in DCM (10 mL) afforded 6 (314 mg, 94%) after purification by chromatography on SiO2(60:40,hexanes/EtOAc). Spectral data are consistent with those reported in the literature.9
R f (hexanes/DCM/acetone/MeOH 6:2:1.5:0.5): 0.36;
1H NMR (CDCl
, 5 MHz): δ 6 ( , J = 8.6, 2.2 Hz, 1H), 7.62 (d, J = 2.0 Hz, 1H), 7.00 (td,
3
J = 7.7, 7.7, 1.2 Hz, 1H), 6.91 (ddd, J = 8.3, 8.3, 1.5 Hz, 1H), 6.89 (d, J = 8.31 Hz, 1H), 6.83 (td,
J = 7.26, 7.26 Hz, 1H), 5.40 (t, J = 5.4, 5.4 Hz, 1H), 4.07, (d, J = 5.4 Hz, 2H), 3.95 (s, 3H), 3.92 (s, 3H), 3.86 (s, 3H), 3.06 (br s, 1H).
7Angew. Chem. Int. Ed. 2012, 51, 3410.
8Chem. Eur. J. 2011, 17, 13877.
9J. Am. Chem. Soc.2013, 135, 6415.
S12
1-(3,4-dimethoxyphenyl)-3-hydroxypropan-1-one (table 1, entry 6, product 1): According to General Procedure B, 6 (0.38 g, 1.14 mmol), DIPEA (0.60 mL, 0.44 g, 3.4 mmol), formic acid (0.13 mL, 0.16 g, 3.4 mmol) and [Ir(ppy)2(dtbbpy)]PF6 ( mg, μmo ) M CN ( mL) afforded S8 (141 mg, 85%) and guaiacol (239 mg, 76%) after purification by chromatography on SiO2 (70:20:5:5 hexanes/DCM/MeOH/acetone).
5.2 mmol Scale One-Pot Reductive Fragmentation of 11 to S8
According to General Procedure C, 11 (1.75 g, 5.22 mmol) in DCM (50.0 mL), silica gel (1.75 g, 100 wt. %), and [4-AcNH-TEMPO]BF4(1.65 g, 5.5 mmol). The vial was capped and the heterogenous mixture was stirred at room temperature until it was complete (as judged by TLC analysis). The reaction mixture was vacuum filtered through a pad of silica on a glass-fritted funnel (pore size C) and an additional 90 mL of DCM (30 mL portions) was used to rinse 6 from the silica. The organic layer was concentrated and then the crude was reacted with DIPEA (2.75 mL, 2.0 g, 16 mmol), formic acid (0.60 mL, 0.73 g, 16 mmol) and [Ir(ppy)2(dtbbpy)]PF6 (2 mg, 2 μmo ) M CN (5 mL) afforded S8 (1.05 g, 95%) and guaiacol (0.65 g, 99%) after purification by chromatography on SiO2 (70:20:5:5 hexanes/DCM/MeOH/acetone).
R f (hexanes/DCM/MeOH/acetone 7:2:0.5:0.5): 0.21;
IR (neat): 3437, 2938, 1667, 1586, 1514, 1464, 1418, 1347, 1264, 1201, 1150, 1021, 888, 808, 766 cm-1;
1H NMR (CDCl
, MHz): δ 59 ( , J = 8.3 Hz, 1H), 7.52 (s, 1H), 6.89 (d, J = 8.3 Hz, 1H),
3
4.02 (m, 2H), 3.95 (s, 3H), 3.93 (s, 3H), 3.19 (t, J =
5.3 Hz, 2H);
13C NMR (CDCl
, 5 MHz): δ 23 5, 29 9, 23 , , 9 9, 58 3, 56 , 56 , 39 8;
3
HRMS (ESI) m/z calculated for C11H15O4+ ([M+1]+
) 211.0965, found 211.0960.
1-(4-(benzyloxy)-3,5-dimethoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol (S3): Substrate S3was synthesized according the literature procedure.7To o ut o of β-hydroxyester7(5.0 g, 9.5 mmol) in THF/H2O (5 mL, 3:1 ratio) was added sodium borohydride
S13
(1.8 g, 47 mmol) in portions. The reaction was allowed to stir at room temperature overnight. The reaction was diluted with ethyl acetate and washed with water and brine, then dried over sodium sulfate. The organic layer was then concentrated and purified by chromatography on SiO2 (60:40, hexanes/EtOAc) afforded S3 (3.8 g, 91%, 2.5:1 diastereomeric ratio). Spectral data are consistent with those reported in the literature.7
R f (hexanes:EtOAc 7:1): 0.10;
1H NMR (CDCl
, 5 MHz): δ 7.47 (d, J = 6.8 Hz, 2H, minor diastereomer), 7.47 (d, J = 6.8 Hz,
3
2H, major diastereomer), 7.33 (m, 2H, minor diastereomer), 7.33 (m, 2H, major diastereomer) 7.29 (d, J = 6.8 Hz, 1H, minor diastereomer), 7.29 (d, J = 6.8 Hz, 1H, major diastereomer), 7.10 (m, 1H, minor diastereomer), 6.95 (m, 3H), 6.67 (s, 0.82H, minor diastereomer), 6.60 (s, 2H, major diastereomer), 4.99 (m, 2H, major diastereomer), 4.97 (d, J= 4.4 Hz, 1.3H, minor diastereomer), 4.16 (m, 1H, major diastereomer), 4.02 (m, 0.6H, minor diastereomer), 3.92 (s, 1.4H, minor diastereomer), 3.90 (s, 3H, major diastereomer), 3.82 (s, 1.83H, minor diastereomer), 3.81 (s, 6H, major diastereomer), 3.66 (m, 1H, major diastereomer), 3.51 (m, 0.5H, minor diastereomer).
1-(4-(benzyloxy)-3,5-dimethoxyphenyl)-3-hydroxy-2-(2-methoxyphenoxy)propan-1-one (S6): According to General Procedure A, S3 (1.0 g, 2.3 mmol), [4-AcNH-TEMPO]BF4 (0.72 g, 2.4 mmol), and silica (1.0 g) in DCM (20 mL) afforded S6 (0.94 g, 94%) after purification by chromatography on SiO2(60:40, hexanes/EtOAc). Spectral data are consistent with those reported in the literature.10
R f (hexanes/EtOAc 4:1): 0.15;
1H NMR (CDCl
, 5 MHz): δ 7.46-7.43 (m, 2H), 7.34-7.31 (m, 2H), 7.32 (s, 2H), 7.02 (ddd, J
3
= 7.8, 7.8, 1.6 Hz, 1H), 6.92 (ddd, J = 8, 1.5, 1.5 Hz, 2H), 6.85 (ddd, J = 7.7, 7.7, 1.5 Hz, 1H), 5.35 (d, J = 5.1, 5.1 Hz, 1H), 5.10 (s, 2H), 4.09-4.07 (m, 2H), 3.82 (s, 6H), 2.93 (dd, J = 6.0, 6.0 Hz, 1H).
10J. Photochem. Photobiol. A 2003, 156, 253.
S14
3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-2-(2-methoxyphenoxy)propan-1-one (7): A solution of S6(150 mg, 0.23 mmol) and pentamethylbenzene (101 mg, 0.69 mmol) in dicholoromethane was cooled to -78 C in a dry ice/acetone bath. To this, BCl3 (450 μL, 1M solution in DCM, 0.45 mmol) was added dropwise.11Th o to t t - 8 C for 30 minutes upon which the reaction was quenched with MeOH. This organic layer was immediately loaded onto celite and purified by chromatography on SiO2 (50:50, hexanes/EtOAc) to afford 7 (75 mg, 95%) as a colorless solid. Spectral data are consistent with those reported in the literature.7
R f (hexanes/EtOAc, 8:2): 0.06;
1H NMR (CDCl
, 5 MHz): δ 4 ( , 2H), ( , J = 8.1, 7.3, 1.5 Hz, 1H), 6.91 (ddd, J =
3
9.5, 8.5, 1.7 Hz, 2H), 6.83 (ddd, J = 7.6, 7.6, 1.5 Hz, 1H), 6.01 (br s, 1H), 5.34 (dd, J = 6.5, 4.3
= 3.2 Hz, 1H), 3.91 (s, 6H), 3.86 (s, 3H).
Hz, 1H), 4.09 (d, J = 5.4 Hz, 1H), 4.08 (d, J
3-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)propan-1-one (table 1, entry 7, product 1): According to General Procedure B, 712(71 mg, 0.19 mmol), DIPEA (0.13 mL, 96 mg, 0.75 mmol), formic acid (28 μL, 34 mg, 0.75 mmol) and [Ir(ppy)2(dtbbpy)]PF6 (1.7 mg, 2 μmo ) MeCN (0.5 mL) afforded S9 (41 mg, 90%) and guaiacol (22 mg, 95%) after purification by chromatography on SiO2(70:10:10:10, hexanes/DCM/MeOH/acetone). Spectral data are consistent with those reported in the literature.12
R f (hexanes/DCM/acetone/MeOH 7:1:1:1) 0.05;
1H NMR (CDCl
, MHz): δ 26 ( , 2H), 5 99 (b s, 1H), 4.03 (q, J = 8.4 Hz, 2H), 3.96 (s,
3
3H), 3.20 (t, J = 5.3 Hz, 2H), 2.67 (t, J = 6.3 Hz, 1H).
3-(3,4-dimethoxyphenyl)-3-ethoxy-2-(2-methoxyphenoxy)propan-1-ol (S10): Compound S10
was made according to literature precedent.13 1 drop of conc. HCl was added a solution of 11
11Synlett 2008, 13, 1977.
12J. Chem. Soc. Pak.2009, 31, 126.
13Holzforschung1991, 45, 37.
S15
(0.75 g, 2.2 mmol) in ethanol (6 mL) and heated to 60°C for 3 hours. The reaction was concentrated and purified by chromatography over SiO2 (75:25, hexanes/EtOAc) to afford S10 (0.68 g, 84% 1:4:1 mixture of diastereomers) as a clear and colorless oil. Spectral data are consistent with those reported in the literature.13
R f (hexanes/EtOAc 3:1): 0.26;
1H NMR (CDCl
, 500 MHz): δ 29 (dd, J = 7.8, 1.5 Hz, 1H, minor diastereomer), 7.02 (m, 1H,
3
minor diastereomer), 6.97-6.89 (m, 3H, major diastereomer, 4H, minor diastereomer), 6.86-6.82 (m, 2H, major diastereomer, 1H, minor diastereomer), 6.75 (ddd, J = 7.7, 7.7, 1.7 Hz, 1H, major diastereomer), 6.50 (dd, J = 7.8, 1.5 Hz, 1H, major diastereomer), 4.53 (d, J = 7.3 Hz, 1H, major diastereomer), 4.50 (d, J = 7.3 Hz, 1H, minor diastereomer), 4.18 (ddd, J = 7.0, 7.0, 3.7 Hz, 1H, minor diastereomer), 4.08 (m, 1H, major diastereomer), 3.93 (br. s., 1H, major diastereomer), 3.89 (s, 3H, minor diastereomer), 3.88 (s, 3H, minor diastereomer), 3.88 (s, 3H, major diastereomer), 3.87 (s, 3H, minor diastereomer), 3.86 (s, 3H, major diastereomer), 3.82 (s, 3H, major diastereomer), 3.52-3.38 (m, 2H, major diastereomer, 2H, minor diastereomer), 3.39 (br. s., 1H, major diastereomer), 3.23 (br. s., 1H, major diastereomer, 1H, minor diastereomer), 1.21 (t, J = 7, 7 Hz, 3H major diastereomer), 1.19 (t, J = 7, 7 Hz, 3H, minor diastereomer).
3-(3,4-dimethoxyphenyl)-3-ethoxy-2-(2-methoxyphenoxy)propanal (8): To a solution of S10 (108 mg, 0.30 mmol) in a 1:1 mixture of DCM/DMSO (2 mL), was added SO3?Py (190 mg, 1.2 mmol) and triethylamine (0.17 mL, 120 mg, 1.2 mmol). This reaction was allowed to stir at room temperature for 3 hours upon which it was diluted with ethyl acetate and washed with five portions of water and one portion of brine. The organic layer was dried over sodium sulfate and concentrated to afford 8 as a yellow oil (97 mg as a 1.5:1 mixture of diastereomers). This was found to be unstable to chromatography and was used directly in the next step.
R f(hexanes/EtOAc 1:3): 0.25;
IR (neat): 3452, 2972, 2933, 1734, 1594, 1502, 1456, 1258, 1118, 1113, 1027, 750 cm-1;
1H NMR (CDCl
, 500 MHz): δ 9 88 ( , J = 2.0 Hz, 1H, minor diastereomer), 9.76 (d, J = 2.9,
3
1H, major diastereomer), 7.12 (d, J = 2.0 Hz, 1H, minor diastereomer), 7.00 (d, J= 2.0, 1H, major diastereomer), 6.97-6.91 (m, 2H, major diastereomer, 2H, minor diastereomer), 6.84 (dd, J = 8.3, 1.5 Hz, 2H, major diastereomer), 6.82 (dd, J = 8.3, 1.5 Hz, 2H, minor diastereomer), 6.75–6.79 (m, 1H, major diastereomer), 6.72–6.68 (m, 1H, major diastereomer, 1H minor diastereomer), 6.53 (dd, J = 8.3, 1.5, 1H, minor diastereomer), 4.77 (d, J = 3.4 Hz, 1H, minor diastereomer), 4.71 (d, J = 5.9 Hz, 1H, major diastereomer), 4.44 (dd, J = 5.9, 2.4 Hz, 1H, major diastereomer), 4.23 (dd, J= 3.2, 2.2 Hz, 1H, minor diastereomer), 3.90 (s, 3H, minor
S16
diastereomer), 3.87 (s, 6H, major diastereomer), 3.87 (3H, minor diastereomer), 3.75 (s, 3H, major diastereomer), 3.74 (s, 3, minor diastereomer), 3.51-3.36 (m, 2H, major diastereomer, 2H, minor diastereomer), 1.19 (t, J = 7.1 Hz, 3H, minor diastereomer), 1.18 (t, J = 7.1 Hz, 3H, major diastereomer);
13C NMR (CDCl
, 25 MHz): δ 2 3 , 2 4, 5 3, 49 , 4 4, 3 2, 3 , 23 6, 23 5,
3
120.9, 120.9, 120.2, 119.8, 118.8, 118.7, 112.4, 112.2, 110.7, 110.7, 110.6, 88.4, 87.0, 82.1, 81.0, 65.2, 64.7, 55.9, 55.9, 55.9, 55.9, 55.6, 55.4, 15.1, 15.0;
) 383.1471, found 383.1461.
HRMS (ESI) m/z calculated for C20H24O6Na+ ([M+Na]+
Fragmentation of Compound 9 (Table 1, Entry 9): According to General Procedure B crude aldehyde 9 (117 mg, 0.33 mmol), DIPEA (0.17 mL, 126 mg, 0.97 mmol), formic acid (0.04 mL, 45 mg, 0.97 mmol) and [Ir(ppy)2(dtbbpy)]PF6 (3.0 mg, 3 3 μmo ) M CN ( 5 mL) ffo ethyl 3,4-dimethoxybenzoate14(21 mg, 30%) and guaiacol (28 mg, 70%) after purification by chromatography on SiO2 (70:20:5:5, hexanes/DCM/MeOH/acetone). Spectral data are consistent with those reported in the literature.
1-(4-methoxyphenyl)ethane-1,2-diol (S1):Substrate S1 was prepared according to a literature procedure.6In a flask, the α-ketoester (1.0 mmol) was dissolved in methanol (5 mL). Then NaBH4(3.0 equiv) was added portion wise. The reaction mixture was stirred at room temperature until the reaction was completed based on TLC monitoring. Upon completion of the reaction, the mixture was acidified using 5.0 M HCl until pH 6. The solvent was then evaporated using rotary evaporator. The residue was dissolved in brine solution and the crude material was extracted using EtOAc. The organic layer was dried using anhydrous Na2SO4, filtered, and the solvent was evaporated. Purification by chromatography on SiO2(25:75, hexanes/EtOAc) afforded S1 (0.17 g, 1.0 mmol, 100%). Spectral data are consistent with those reported in the literature.15
R f (EtOAc/hexanes 3:2): 0.18;
14Org. Biomol. Chem.2012, 10, 506.
15J. Am. Chem. Soc.2010, 132, 14409.
S17
1H NMR (CDCl
, 4 MHz): δ 7.30 (d, J = 8.4 Hz, 2H), 6.90 (d, J = 8.8 Hz, 2H), 4.83–4.74 (m,
3
1H), 3.81 (s, 3H), 3.76–3.63 (m, 2H), 2.51 (br s, 1H), 2.12 (br s, 1H).
2-(benzyloxy)-1-phenylethanol (S2):Substrate S2was prepared according to a literature procedure.16To a solution of 2-(benzyloxy)acetaldehyde (4) (20 mmol) in THF (40 mL) was added PhMgBr 3.0 M in Et2O (22 mmo q ) t C. After the mixture was stirred for 10 min at 0 °C, the mixture was warmed up to room temperature and was stirred for another 2 hours. The mixture was carefully quenched with saturated aq. NH4C t C, and the organic layer was separated and aqueous layer was extracted with Et2O twice. The combined organic layer was washed with brine and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure and the residue was purified by chromatography on SiO2(90:10, hexanes/EtOAc) to afford S2 (3.9 g, 17 mmol, 85%) as a clear and colorless oil. Spectral data are consistent with those reported in the literature.16
R f (EtOAc/hexanes 1:3): 0.48;
1H NMR (CDCl
, 4 MHz): δ 7.40–7.29 (m, 10H), 4.98 (dd, J = 9.0, 3.3 Hz, 1H), 4.63 (d, J =
3
12.0 Hz, 1H), 4.59 (d, J = 12.0 Hz, 1H), 3.67 (dd, J = 9.6, 3.3 Hz, 1H), 3.54 (dd, J = 9.6, 9.0 Hz,
1H), 2.87 (br s, 1H).
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethanol (9):Substrate 9was prepared according to a literature procedure.1 A round bottom flask was charged with 1 (1.7 g, 6.2 mmol), THF (28 mL), and water (7 mL). Sodium borohydride (0.47 g, 12.4 mmol) was added portion-wise to maintain a gentle evolution of gas over 5 minutes, after which the reaction mixture was stirred for 3 h at room temperature. The reaction was quenched with saturated aqueous NH4Cl (50 mL) and then the reaction mixture was diluted with water (50 mL). The aqueous portion was extracted with Et2O (3 x 50 mL). The combined organic extracts were washed twice with brine, dried over MgSO4, filtered, and concentrated in vacuo. Purification by chromatography on SiO2 (75:25, hexanes/EtOAc) afforded 9 (1.4 g, 5.0 mmol, 80%) as a clear and colorless oil.
R f (EtOAc/hexanes 1:1): 0.43;
16 J. Am. Chem. Soc.2012, 134, 10329.
S18
IR (neat): 3454, 2933, 2836, 1612, 1593, 1505, 1455, 1250, 1177, 1124, 1027, 832, 745 cm-1;
1H NMR (CDCl
, 4 MHz): δ 7.37 (d, J = 9.0 Hz, 2H) 7.03–6.88 (m, 6H), 5.06 (d, J = 9.0 Hz,
3
1H), 4.16 (dd, J = 10, 2.8 Hz, 1H), 4.01–3.93 (m, 1H), 3.90 (s, 3H), 3.82 (s, 3H), 3.38 (br s, 1H); 13C NMR (CDCl
, 175 MHz): δ 159.3, 150.0, 148.0, 131.7, 127.5, 122.4, 121.0, 115.8, 113.8,
3
111.9, 76.1, 71.9, 55.8, 55.2;
HRMS (ESI) m/z calculated for C16H18O4+ ([M+1]+
) 292.1543, found 292.1537.
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)propane-1,3-diol (10):Substrate 10 was prepared according to a literature procedure.9 Substrate 5 (1.1 g, 3.5 mmol) was dissolved in the mixture of THF:H2O (5:1) (25 mL), and sodium borohydride (0.26 g, 7.0 mmol) was added portion-wise to maintain a gentle evolution of gas. Then, the mixture was stirred for 6 h at room temperature. The reaction mixture was quenched with saturated aqueous NH4Cl (50 mL) and diluted with 30 mL water. The aqueous portion was extracted with ethyl acetate (3 × 30 mL). The organic portions were combined, dried over MgSO4, filtered and concentrated in vacuo. Purification by chromatography on SiO2(40:60, hexanes/EtOAc) afforded 10 as a mixture of diastereomers (0.75: 1) and a thick colorless oil (0.80 g, 2.6 mmol, 75%). Spectral data are consistent with those reported in the literature.8
R f (EtOAc/hexanes 3:2): 0.31;
1H NMR (CDCl
, 4 MHz): δ 7.38 (d, J = 7.8 Hz, 2H, major diastereomer), 7.32 (d, J = 7.4 Hz,
3
2H, minor diastereomer), 7.14 (d, J = 7.6 Hz, 1H, major diastereomer), 7.11–7.04 (m, 1H, both
diastereomers), 7.01–6.87 (m, 4H, major diastereomer, 5H, minor diastereomer), 5.04–4.98 (m,
1H, both diastereomers), 4.20–4.14 (m, 1H, minor diastereomer), 4.07–4.01 (m, 1H, major
diastereomer), 3.96–3.87 (m, 1H, minor diastereomer), 3.93 (s, 3H, major diastereomer), 3.90 (s,
3H, minor diastereomer), 3.82 (s, 3H, both diastereomers), 3.70–3.59 (m, 2H, major
diastereomer, 1H, minor diastereomer), 3.51–3.39 (m, 1H, both diastereomers), 2.78–2.67 (m, 1H, both diastereomers).
2-hydroxy-1-(4-methoxyphenyl)ethanone (S4):According to General Procedure A, S1 (0.17 g, 1.0 mmol), [4-AcNH-TEMPO]BF4(0.32 g, 1.05 mmol), and silica (0.17 g) in DCM (10 mL)
S19
afforded S4 (0.16 g, 95%) after purification by chromatography on SiO2 (60:40, hexanes/EtOAc) as a colorless solid. Spectral data are consistent with those reported in the literature.17
R f (EtOAc/hexanes 1:3): 0.13;
1H NMR (CDCl
, 4 MHz): δ 92 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.4 Hz, 2H), 4.83 (s, 2H),
3
3.90 (s, 3H), 3.57 (br s, 1H).
2-(benzyloxy)-1-phenylethanone (S5):According to General Procedure A, S2(0.23 g, 1.0 mmol), [4-AcNH-TEMPO]BF4(0.32 g, 1.05 mmol), and silica (0.23 g) in DCM (10 mL) afforded S5 (0.21 g, 95%) after purification by chromatography on SiO2 (75:25, hexanes/EtOAc) as a colorless solid. Spectral data are consistent with those reported in the literature.18
R f (EtOAc/hexanes 3:2): 0.77;
1H NMR (CDCl
, 4 MHz): δ 7.93 (d, J = 7.6 Hz, 2H), 7.59 (t, J = 7.6 Hz, 1H), 7.47 (t, J = 8.0
3
Hz, 2H), 7.43–7.27 (m, 5H), 4.77 (s, 2H), 4.71 (s, 2H).
17J. Organomet. Chem. 2009, 694, 3452.
18J. Org. Chem.2011, 76, 3576.
S20
木质素的测定方法研究进展
本文由dylan_may贡献 pdf文档可能在WAP端浏览体验不佳。建议您优先选择TXT,或下载源文件到本机查看。 第 41 卷 3 期第 2007 年 6 月 河南农业大学学报 Journal of Henan A gricultural U niversity Vol 41 No. 3 . Jun. 2007 文章编号 : 1000 - 2340 ( 2007 ) 03 - 0356 - 07 木质素的测定方法研究进展 苏同福 ,高玉珍 ,刘 ,周 ,宫长荣霞斌 1 1 1 2 1 ( 1. 河南农业大学 ,河南郑州 450002; 2. 黄河中心医院药剂科 ,河南 郑州 450003 ) 摘要 : 对木质素的制备、总量的测定及其结构和分子量的测定等进行了综述 , 并分析了这些测定方法存在的问题 ,指出了将太赫兹技术应 用于木质素测定的前景 . 关键词 : 木质素 ; 降解 ; 太赫兹中图分类号 : Q 539; O 636. 2 文献标识码 : A Rev iew of D eterm ina tion of L ign in SU Tong2fu , GAO Yu 2zhen , L I Xia , ZHOU B in , GONG Chang2rong U ( 1. Henan Agricultural University, Zhengzhou 450002, China; 1 1 1 2 1 2. Pharmacy of yellow R iver Central Hosp ital, Zhengzhou 450003, China ) Abstract: Testing methods for total lignin, p reparation of lignin, structures and molecular weight, are introduced in this article. Problem s existing in these testing methods are analysed and the p rospects of the terahertz technology app lication to lignin analysis are pointed out . Key words: lignin; decompose; terahertz 木质素 ,又称为木素 , 广泛地存在于木材与禾本植物体内 , 通常认为是植物体在次生代谢合成的 ,在植物体内具有机械支持、防止生物降解、输送水分等功能 . 木质素的化学组成是苯丙烷类物质 (包括对羟基苯丙烷、—邻甲氧基苯丙烷以及 4 —羟基—3, 5 —二甲氧
去除木质素
目前利用木质纤维素生物质的方法主要是在纤维素转化阶段之前利用溶剂或化学品脱除木质素的方法,秸秆等木质纤维素原料的利用思路如下: 利用溶剂或化学品溶解木质素的过程往往需要高温处理,一旦降温,木质素即沉淀析出,易造成浆液浓稠,设备结垢的难题。超临界方法作为一种绿色化学的处理工艺,目前已经在木质纤维素的预处理过程中有所应用,主要原理是在超临界状态下利用CO2等溶剂及改性剂的作用破坏纤维素与半纤维素、木质素的链接,达到提高木质纤维素产糖率的目的。可以查询到的专利有:一种以棉籽壳为原料制备纤维素类化合物的方法(CN103122034A,2013年5月公布);一种玉米秸秆预处理方法(CN101565725A,2009年10月);从木质纤维素生物质生产木质素(CN103502320A,2014年1月公布);从木质纤维素生物质生产木质素(CN103502383A,2014年1月公布)等。综合以上处理方法,其主要工艺流程可归纳如下: (a)样品处理; 粉碎机处理样品,使样品的表面积尽可能增加。 (b)木质素去除; 利用醇(甲醇,乙醇,丁醇,戊醇)、超临界CO2(31度,1072 psig)、亚临界水(250-280度)、超临界水(>374度,>221 bar)的一种或多种作为反应萃取溶剂。采用间歇式或连续式的方法处理木质纤维素样品。有报道采用流量20g/min CO2,33%的戊醇水溶液作为萃取剂,在180度,15MPa的条件下处理秸秆后,其最终产糖率由8%提高到93%,木质素去除率达到90%。 为了防止木质素沉降聚集,制备木质素微粒(粒度范围50-500微米),在脱除木质素的过程中有专利提出了采用多级降温降压的措施。
