Freeze casting of aqueous alumina slurries with glycerol for porous ceramics

Freeze casting of aqueous alumina slurries with glycerol for porous ceramics Yumin Zhang a,Luyang Hu a,*,Jiecai Han a,Zehui Jiang b
a Center for Composite Materials,Harbin Institute of Technology,Harbin150001,China
b Department of Physics,Harbin Institute of Technology,Harbin150001,China
Received28June2009;received in revised form12August2009;accepted24September2009
Available online29October2009
Abstract
Experiments have been performed to show that the mechanical properties of alumina porous ceramics may be improved by introducing glycerol into the raw slurries and then casting them under a constant cooling rate.The effects of glycerol on the freeze casting process and thereby on the microstructure and mechanical properties of porous ceramics obtained are investigated.It is shown that the addition of glycerol will increase both the slurry viscosity and sample sintered density.SEM images for microstructure of the?nal ceramics reveal that a good connection between ceramic lamellae has been promoted.This connection makes as-prepared porous ceramics obtain high mechanical properties.For the30vol.% alumina slurry with glycerol,the axial and radial compression strengths reach to,respectively,255.1MPa and105.8MPa.
Crown Copyright#2009Published by Elsevier Ltd and Techna Group S.r.l.All rights reserved.
Keywords:B.Microstructure-?nal;D.Al2O3;Freeze casting;Porous
1.Introduction
Porous ceramic as a technological important material posses
a wide range of applications,such as separation materials[1],
catalyst supports[2,3],and implantable bioceramics[4–6].
Various methods for the manufacturing of such materials have
been developed in the past years.Recently,the freeze casting
technique,as a novel method,causes much attention,due to its
simple operation,widely controllable porosity and environ-
mental friendliness[4–19].In this technique,the dispersed
slurry is poured into a mold and then frozen by layer by layer
solidi?cation mode[4,17–19]or unidirectional solidi?cation
mode[5–16].During the freezing process,the particles are
expelled from the moving solidi?cation front,and pile up
between the vehicle crystals until the process completed.
Subsequently,sublimation of the vehicle is made to eliminate
the drying stresses,avoiding shrinkage,cracks and warping of
the green parts that generally existing in the normal drying.
Finally,the porous materials with a lamellar or/and columnar
continuous microstructure are obtained by sintering.
However,as a special unidirectional solidi?cation,the
constant cooling rate freezing method,which can achieve a
homogeneous lamellae spacing and lamellae thickness by
controlling a constant temperature gradient,has not arisen
considerable investigation.In addition,freeze casting of
aqueous slurries for porous ceramics mainly focuses on the
use of slurry without antifreeze agent[6–13].Little attention
has been paid to the effect of antifreeze agent[20,21].In this
work,we introduce glycerol,as an antifreeze agent,into the
slurry,to fabricate porous ceramics by a constant cooling rate
process and investigate the effect of glycerol on the freeze
casting process.Herein,alumina is used as model material.
2.Experimental procedure
First,distilled water,a small amount(1.2wt.%of the
alumina powder)of dispersant(Darvan7-N,R.T.Vanderbilt
Co.,Norwalk,CT),binder(polyvinylalcohol,1wt.%of the
alumina powder)and glycerol(10wt.%of solvent)were mixed
in the milling container.Then,an appropriate amount of
alumina powder(99.99%a-Al2O3,mean particle size0.4m m,
Dalian Luming Nanometer Material Co.,Ltd.,Dalian,China)
was added into the mixed solutions and ball-milled for24h
with zirconia balls and de-aired by stirring in a vacuum
desiccator.Here slurries with20vol.%and30vol.%solid
content were chosen for the fabrication of porous ceramics.For
the purpose of comparison,the slurries containing same solid
content without glycerol were also prepared.
https://www.wendangku.net/doc/8714588620.html,/locate/ceramint
Available online at https://www.wendangku.net/doc/8714588620.html,
Ceramics International36(2010)617–621
*Corresponding author.Tel.:+8645186412236;fax:+8645186412236.
E-mail address:huluyang2005@https://www.wendangku.net/doc/8714588620.html,(L.Hu).
0272-8842/$36.00.Crown Copyright#2009Published by Elsevier Ltd and Techna Group S.r.l.All rights reserved.
doi:10.1016/j.ceramint.2009.09.036
The mixed slurries were poured into polyethylene molds (20mm diameter,15mm long)and placed on refrigeration equipment,similar to that designed by Deville et al.[11–13].The cooling rate is kept at 68C/min to induce the unidirectional solidi?cation of the slurries from the bottom to the top.The frozen samples were then freeze-dried for 48h to remove the solvent.All green parts were sintered in air for 2.5h at 15508C
with a constant rate of 58C/min,followed by cooling naturally in furnace to room temperature.
The viscosity of the slurries was characterized by rheometer with a concentric-cylinder geometry (Physica MCR300,Anton Paar GmbH,Graz,Austria).Zeta potential measurements were conducted with a Zetasizer 3000(Malvern Instruments Ltd.,Worcester,UK).The as-prepared products were coated with a thin layer of gold and characterized in a scanning electron microscope (FEI Quanta 200,FEI Company,Hillsboro,US).The apparent density and porosity of sintered bodies were measured using the Archimedes method.Samples of 4mm ?4mm ?5mm were cut off from the sintered bodies,and were loaded at a testing machine (Instron 5569,Instron corp.,Canton,US)to test the compressive strengths,with a crosshead speed of 1mm/min.In order to obtain the average value,more than four samples of each measurement were
chosen.
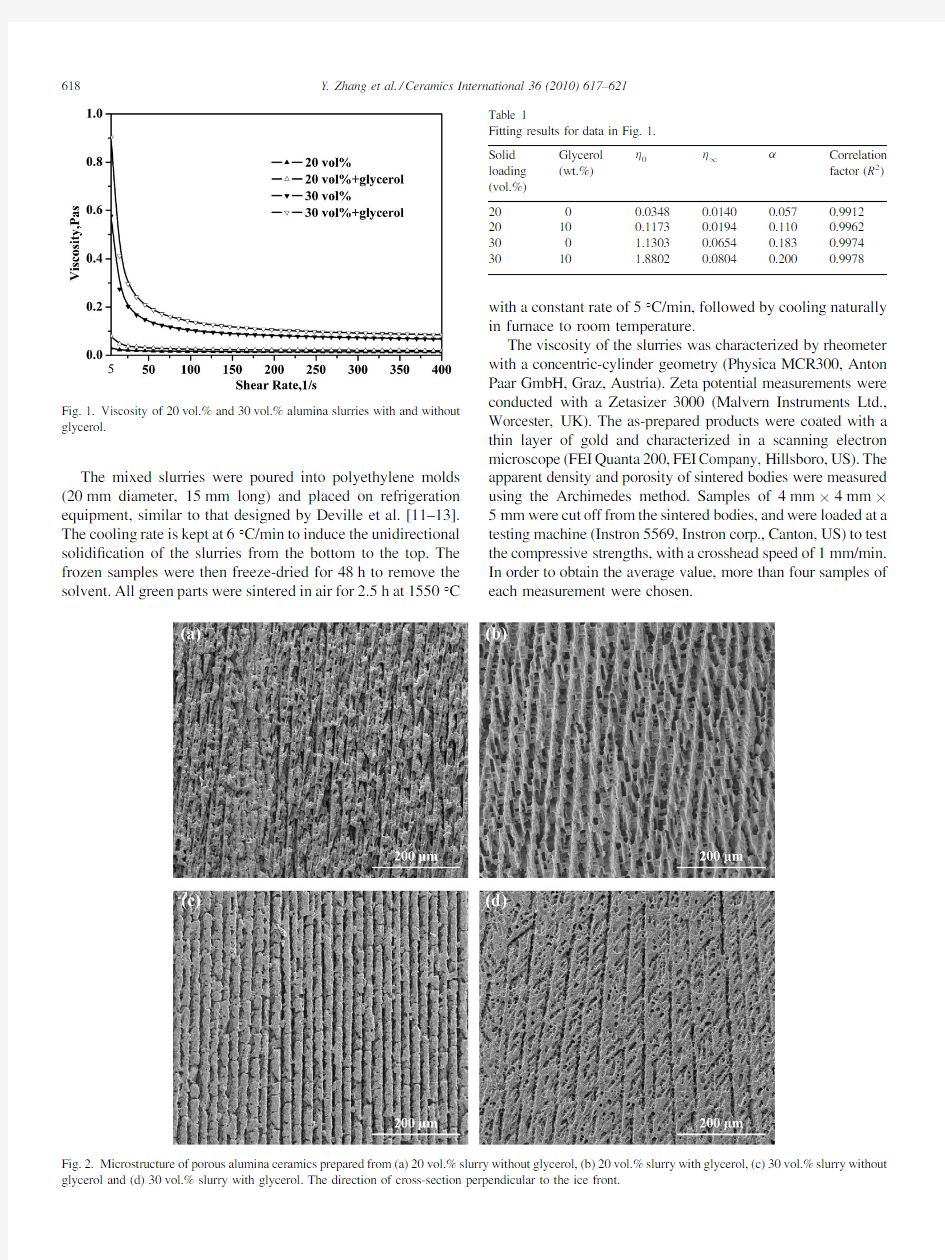
Fig.1.Viscosity of 20vol.%and 30vol.%alumina slurries with and without
glycerol.
Fig.2.Microstructure of porous alumina ceramics prepared from (a)20vol.%slurry without glycerol,(b)20vol.%slurry with glycerol,(c)30vol.%slurry without glycerol and (d)30vol.%slurry with glycerol.The direction of cross-section perpendicular to the ice front.
Table 1
Fitting results for data in Fig.1.Solid loading (vol.%)Glycerol (wt.%)h 0
h 1
a
Correlation factor (R 2)2000.03480.01400.0570.991220100.11730.01940.1100.9962300 1.13030.06540.1830.997430
10
1.88020.08040.200
0.9978
Y.Zhang et al./Ceramics International 36(2010)617–621
618
3.Results and discussion
Fig.1shows the viscosity of20vol.%and30vol.%alumina slurries with and without glycerol.It is clear that the slurries with glycerol always have a higher viscosity than those without glycerol.With the increase of shear rate,all of the slurries exhibit shear-thinning behavior.
Measurements on zeta potential show that alumina particles are negatively charged with the zeta potential aboutà23.0mV andà23.9mV for slurries with and without glycerol, respectively.This suggests that the electrostatic interaction between particles is not the main reason leading to the enhancement of viscosity.Lu et al.[22,23]supposed that the carboxyl groups can interact with the hydroxyl groups to form link structure by hydrogen bonding.Therefore,the differences of viscosity between slurries with and without glycerol perhaps come from the effect of the links.The more the links,the easier are the tanglement between the links and the greater are the viscosity values.As a result,the higher viscosities exist in the slurries with glycerol(Fig.1).
To describe the rheological behavior of non-Newionian ?uids,Cross[24]establishes a simple expression:
h?h1t
h0àh1
1tag
(1)
where h0and h1are limiting viscosity at zero and in?nite rate of shear,respectively.h is the slurry viscosity and vary with shear rate g.a is a parameter with the rupture of linkages. According to the measured data in Fig.1,the?tting variables are evaluated in Table1.Here,n is a common exponent10/9,
Table2
Properties of sintered porous alumina ceramics.
Solid
loading(vol.%)Glycerol
(wt.%)
Apparent
density(g/cm3)
Porosity
(%)
Open porosity
(%)
Axial compression
strength(MPa)
Radial compression
strength(MPa)
200 1.398?0.01864.9?0.563.3?1.096.4?4.029.7?1.7 2010 1.431?0.02564.1?0.663.1?1.3127.6?9.334.1?2.3 300 2.019?0.02649.3?0.744.9?1.4194.2?12.441.5?3.2
3010 2.063?0.01648.2?0.445.9?0.9255.1?14.1105.8?
6.7
Fig.3.SEM micrographs of porous alumina ceramics,which are prepared by using:(a)20vol.%slurry without glycerol,(b)20vol.%slurry with glycerol,(c) 30vol.%slurry without glycerol and(d)30vol.%slurry with glycerol.The direction of fracture parallel to the ice front.
Y.Zhang et al./Ceramics International36(2010)617–621619
different from the recommended value2/3by Cross paper[24]. This difference is perhaps due to the in?uence of macromole-cules links on slurry systems.In addition,basing on Cross’s analysis,a high a value implies there is a large shear dependent contribution to structural breakdown[24].In our experiments, a for the slurries containing glycerol always takes a larger value than those not(Table1),which indirectly provides support for the existence of more links in the alumina slurry with glycerol.
The addition of glycerol changes the freezing behavior of water.For example,the volumetric expansion of water will decrease from9%to7.4%by volume at10wt.%glycerol in water[25].Similarly,the addition of glycerol to the alumina slurries will reduce slurries expansion in the freezing process, leading to a slight increase of the sintered density of as-prepared porous ceramics(Table2).
Measurements on the compression strength of porous ceramics show that those prepared from the slurries containing glycerol posses a higher strength(Table2).For20vol.%and 30vol.%alumina slurries with glycerol,the axial(parallel to growth direction of ice crystals)strengths are127.6?9.3MPa and255.1?14.1MPa,respectively.While the radial(perpen-dicular to growth direction of ice crystals)strength for30vol.% alumina slurries with glycerol also reaches as high as 105.8?6.7MPa.Therefore,the preparation of porous alumina ceramics using slurries with glycerol is bene?cial to load-bearing biological application[6].
This enhancement of compression strength may be attributed to the improving on the connection between ceramic lamellae.The typical microstructures of porous ceramics prepared from the slurries containing glycerol or not are illustrated in Figs.2and3.Obviously there exists a good connection between lamellar architectures in the ceramics from the slurries with glycerol(Fig.2(b)and(d)).
In the parallel to directions of the ice front,cell-like pore structure is observed in ceramics from the20vol.%alumina slurries containing glycerol or not(Fig.3(a)and(b)).The cell sizes are measured by an intercept method as15–20m m and5–18m m,respectively.It is worthy to note that the addition of glycerol obviously improves the homogeneity of cell wall (Fig.3(a)and(b)).This may lead to the enhancement of axial and radial compression strength(Table2).In addition,when the solid loading of the slurry containing no glycerol is increased to 30vol.%,the cell-like structure converts into a lamellar one (Fig.3(a)and(c)),while dendritic like features protrude on the surface of the pore walls are still maintained(Fig.4).However, for the ceramic from slurry with glycerol,the microstructure transition does not occur.When solid loading of such slurry increases,the smaller pore exhibits in porous ceramics.
For the slurries containing same solid content,the glycerol concentration is the solely variable parameter.Thus,the difference of microstructure of sintered samples originates from the in?uence of glycerol on the freezing process.In that process,all of solutes are separated from and piled up around the growing pure ice crystals.The equilibrium among the diffusion rate of glycerol,ice crystal growth rate and the alumina particle ejecting rate,combined with a high heat transfer of the slurries with glycerol[25],gives rise to a different ceramic lamellae thickness and interlayer distance compared with the sample(Fig.2)from slurry without glycerol. On the other hand,the addition of glycerol changes the hydrogen bonding of water.It is known that as the glycerol concentration increases from0wt.%to10wt.%,the average number of hydrogen bonding/water in bulk water decreased from3.9to2.4[26].The more water molecules are bound in the ?rst hydration shell around the polar groups of glycerol.This disrupts the complete crystallization of ice,leading to a localized amorphous structure[17].In addition,high slurry viscosity,on some extent,also inhibits the expelling of alumina particles from the growing ice crystals.These related factors together increase the connection between lamellae and improve compression strength of porous ceramics.
4.Conclusions
This study demonstrated the effect of glycerol on freeze casting of aqueous alumina slurries for porous ceramics.Our experiment results indicated that the addition of glycerol into aqueous alumina slurries will increase their viscosity and decrease their volume expansion.Interaction between glycerol and other components in the slurries promotes a good connection between lamellae and thus improve the
mechanical Fig.4.SEM micrographs of dendritic like features on pore walls.Parts(a)and (b)are magni?ed images of Fig.3(a)and(c),respectively.
Y.Zhang et al./Ceramics International36(2010)617–621 620
properties of porous ceramics.For the ceramics prepared from 30vol.%alumina slurries,31.4%and154.9%increment for the axial and radial compression strength can be achieved.
Acknowledgment
This work was supported by National High Technology Research and Development Program of China(no. 2008AA120803).
References
[1]S.Kitaoka,Y.Matsushima,C.Chen,H.Awaji,Thermal cyclic fatigue
behavior of porous ceramics for gas cleaning,Journal of the American Ceramic Society87(5)(2004)906–913.
[2]R.Moene,M.Makkee,J.A.Moulijn,High surface area silicon carbide as
catalyst support characterization and stability,Applied Catalysis A Gen-eral167(2)(1998)321–330.
[3]C.Pham-Huu,C.Bouchy,T.Dintzer,G.Ehret,C.Estournes,M.J.Ledoux,
High surface area silicon carbide doped with zirconium for use as catalyst support.Preparation,characterization and catalytic application,Applied Catalysis A General180(1–2)(1999)385–397.
[4]B.H Yoon,Y.H.Koh,C.S.Park,H.E.Kim,Generation of large pore
channels for bone tissue engineering using camphene-based freeze cast-ing,Journal of the American Ceramic Society90(6)(2007)1744–1752.
[5]B.H.Yoon,W.Y.Choi,H.E.Kim,J.H.Kim,Y.H.Koh,Aligned porous
alumina ceramics with high compressive strengths for bone tissue engi-neering,Scripta Materialia58(7)(2008)537–540.
[6]S.Deville,E.Saiz,A.P.Tomsia,Freeze casting of hydroxyapatite scaf-
folds for bone tissue engineering,Biomaterials27(32)(2006)5480–5489.
[7]T.Fukasawa,Z.Y.Deng,M.Ando,T.Ohji,Y.Goto,Pore structure of
porous ceramics synthesized from water-based slurry by freeze-dry process,Journal of Material Science36(10)(2001)2523–2527.
[8]T.Fukasawa,M.Ando,T.Ohji,S.Kanzaki,Synthesis of porous ceramics
with complex pore structure by freeze-dry processing,Journal of the American Ceramic Society84(1)(2001)230–232.
[9]T.Fukasawa,Z.Y.Deng,M.Ando,T.Ohji,S.Kanzaki,Synthesis of porous
silicon nitride with unidirectionally aligned channels using freeze-drying process,Journal of the American Ceramic Society85(9)(2002)2151–2155.
[10]H.Zhang,I.Hussain,M.Brust,M.F.Butler,S.P.Rannard,A.I.Cooper,
Aligned two-and three-dimensional structures by directional freezing of polymers and nanoparticles,Nature Materials4(10)(2005)787–793.[11]S.Deville,E.Saiz,R.K.Nalla,A.P.Tomsia,Freezing as a path to build
complex composites,Science311(5760)(2006)515–518.
[12]H.Zhang,A.I.Cooper,Aligned porous structures by directional freezing,
Advanced Materials19(11)(2007)1529–1533.
[13]S.Deville,E.Saiz,A.P.Tomsia,Ice-templated porous alumina structures,
Acta Materialia55(6)(2007)1965–1974.
[14]Z.Y.Deng,H.R.Fernandes,J.M.Ventura,S.Kannan,J.M.F.Ferreira,
Nano-TiO2-coated unidirectional porous glass structure prepared by freeze drying and solution in?ltration,Journal of the American Ceramic Society90(4)(2007)1265–1268.
[15]L.Ren,Y.P.Zeng,D.Jiang,Fabrication of gradient pore TiO2sheets by a
novel freeze-tape-casting process,Journal of the American Ceramic Society90(9)(2007)3001–3004.
[16]B.H.Yoon,C.S.Park,H.E.Kim,Y.H.Koh,In situ synthesis of porous
silicon carbide(SiC)ceramics decorated with SiC nanowires,Journal of the American Ceramic Society90(12)(2007)3759–3766.
[17]S.W.So?e,F.Dogan,Freeze casting of aqueous alumina slurries with
glycerol,Journal of the American Ceramic Society84(7)(2001)1459–1464.
[18]K.Araki,J.W.Halloran,Porous ceramic bodies with interconnected pore
channels by a novel freeze casting technique,Journal of the American Ceramic Society88(5)(2005)1108–1114.
[19]L.Ren,Y.Zeng,D.Jiang,Preparation of porous TiO2by a novel freeze
casting,Ceramics International35(3)(2009)1267–1270.
[20]Q.Fu,M.N.Rahaman,F.Dogan,B.S.Bal,Freeze casting of porous
hydroxyapatite scaffolds.I.Processing and general microstructure,Jour-nal of Biomedical Materials Research Part B:Applied Biomaterials86(1) (2008)125–135.
[21]E.Munch,E.Saiz,A.P.Tomsia,S.Deville,Architectural control of freeze-
cast ceramics through additives and templating,Journal of the American Ceramic Society92(7)(2009)1534–1539.
[22]K.Lu,C.S.Kessler,Optimization of a nanoparticle suspension for freeze
casting,Journal of the American Ceramic Society89(8)(2006)2459–2465.
[23]K.Lu,Microstructural evolution of nanoparticle aqueous colloidal sus-
pensions during freezing casting,Journal of the American Ceramic Society90(12)(2007)3753–3758.
[24]M.M.Cross,Rheology of non-Newtonian?uids:a new?ow equation for
pseudoplastic systems,Journal of Colloid Science20(5)(1965)417–437.
[25]C.S.Miner,N.N.Dalton,Glycerol,Reinhold Publishing Corporation,
New York,1953,pp.238–449.
[26]J.L.Dashnau,N.V.Nucci,K.A.Sharp,J.M.Vanderkooi,Hydrogen
bonding and cryoprotective properties of glycerol/water mixtures,Journal of Physical Chemistry B110(27)(2006)13670–13677.
Y.Zhang et al./Ceramics International36(2010)617–621621
Cleanert Alumina N中性氧化铝萃取柱,1000mg_6mL,30支_包
Cleanert Alumina N中性氧化铝萃取柱,1000mg/6mL,30支/包 产品包装尺寸 产品参数 图文介绍 Agela Technologies 吸附型萃取柱
·Cleanert Florisil是一种高选择性的吸附剂。这种吸附剂主要有三种成分组成,二氧化硅(84%),氧化镁(15.5%)和硫酸钠(0.5%)。是一种效果良好,成本经济的常用固相萃取填料。特定为AOAC,EPA等方法设计,用于农药残留的净化﹑分离、内分泌物及油脂的分离、PCBs,PAHs,烃类中含氮化合物和抗生素物质的分离等。常用于农残分析中去除色素,为NY761分析方法中必备的样品前处理小柱。 ·Cleanert PestiCarb采用新型碳黑材料(球形)为填料,具有高净化效果,高回收率和高重现性的优良特性,广泛应用于农残分析中,特别是蔬菜水果等色素较高的样品的前处理中。相当于Envi carb填料。常用于农残分析中去除色素等杂质。 ·Cleanert Alumina N 中性氧化铝萃取柱pH=7.5;强极性吸附剂。表面呈中性,容易保留杂环类(含氮,磷,硫基),芳香烃和有机胺等富电子化合物。经过特殊去活处理,以保证样品的前处理。应用于维生素,抗菌素,芳香油,酶,糖苷,激素等的样品前处理。广泛用于苏丹红和孔雀石绿的样品前处理。 ·Cleanert Alumina A 酸性氧化铝萃取柱pH=4.5,可作为强极性吸附和中等阳离子交换剂。经过特殊去活处理,以保证样品的回收率。可做为中等阳离子交换剂。 ·Cleanert Alumina B (碱性氧化铝萃取柱),pH=10,经过特殊去活处理,以保证样品回收率。可用于除去有机酸,酚类等。
alumina ceramic
Properties of Alumina/Aluminum Oxide (Al2O3): Very good electrical insulation Moderate to extremely high mechanical strength Very high compressive strength High hardness Moderate thermal conductivity High corrosion and wear resistance Good gliding properties Low density Operating temperature without mechanical load 1,000 to 1,500°C. food compatible Applications of Aluminum Oxide (Al2O3): Examples of applications for aluminum oxide Al2O3 advanced ceramics are heavy-duty forming tools, substrates and resistor cores in the electronics industry, tiles for wear protection and ballistics, thread guides in textile engineering, seal and regulator discs for water taps and valves, heat-sinks for lighting systems, protection tubes in thermal processes or catalyst carriers for the chemicals industry.
Cleanert Alumina A酸性氧化铝,1000mg_6mL,30支_包
Cleanert Alumina A酸性氧化铝,1000mg/6mL,30支/包 产品包装尺寸 产品参数 图文介绍 Agela Technologies 吸附型萃取柱
·Cleanert Florisil是一种高选择性的吸附剂。这种吸附剂主要有三种成分组成,二氧化硅(84%),氧化镁(15.5%)和硫酸钠(0.5%)。是一种效果良好,成本经济的常用固相萃取填料。特定为AOAC,EPA等方法设计,用于农药残留的净化﹑分离、内分泌物及油脂的分离、PCBs,PAHs,烃类中含氮化合物和抗生素物质的分离等。常用于农残分析中去除色素,为NY761分析方法中必备的样品前处理小柱。 ·Cleanert PestiCarb采用新型碳黑材料(球形)为填料,具有高净化效果,高回收率和高重现性的优良特性,广泛应用于农残分析中,特别是蔬菜水果等色素较高的样品的前处理中。相当于Envi carb填料。常用于农残分析中去除色素等杂质。 ·Cleanert Alumina N 中性氧化铝萃取柱pH=7.5;强极性吸附剂。表面呈中性,容易保留杂环类(含氮,磷,硫基),芳香烃和有机胺等富电子化合物。经过特殊去活处理,以保证样品的前处理。应用于维生素,抗菌素,芳香油,酶,糖苷,激素等的样品前处理。广泛用于苏丹红和孔雀石绿的样品前处理。 ·Cleanert Alumina A 酸性氧化铝萃取柱pH=4.5,可作为强极性吸附和中等阳离子交换剂。经过特殊去活处理,以保证样品的回收率。可做为中等阳离子交换剂。 ·Cleanert Alumina B (碱性氧化铝萃取柱),pH=10,经过特殊去活处理,以保证样品回收率。可用于除去有机酸,酚类等。
铝产品 Products
产品Products 铝锭Aluminum Ingot 氧化铝alumina ,aluminum oxide 氢氧化铝Hydrogen alumina 电解铝electrolyse aluminum 镀铝aluminum plating 铝板Aluminum Board/plate/sheet 花纹铝板Aluminum embossed sheet 彩铝板colored Aluminum plate 纯铝板pure Aluminum plate 复合铝板clad aluminium 硬铝板duralumin sheet 铝合金Aluminum alloy 锻用铝合金aluminum alloy for temper 铝铜合金aluminum copper alloy 铝基合金aluminum base alloy 铝铁合金aluminum iron alloy 铝镁合金aluminum magnesium alloy 铝镍合金aluminum nickel alloy 铝硅合金aluminum silicon alloy 铝基硅镁合金anticorodal 耐蚀铝合金corrosion-proof/ corrosion- resistant/ corrosion resisting aluminum alloy 铝黄铜aluminum brass 铝青铜aluminum bronze 高铝砖alumina brick 铝合金型材aluminum alloy sections 铝合金线Aluminum alloy wire 铝型材Aluminum profile 铝棒Aluminum stick/rod 铝圆棒Aluminum round bar 铝箔Aluminum foil 铝卷Aluminum roll 铝带/铝条Aluminum strip 铝线Aluminum thread/wire 钢芯铝绞线aluminum cable steel reinforcing 铝管Aluminum pipe/tube 精拉铝管finishing Aluminum tube 伸缩铝管extension Aluminum tube 挤压铝管extruded aluminum pipe 铝管避雷器aluminum cell lightning arrester 挤压铝窗台extruded aluminum sill 铝斜坡Aluminum Ramps 铝曲管bended aluminum tube
铝行业的一些常用英语词汇
铝行业的一些常用英语词汇 产品 Products 铝锭Aluminum Ingot 氧化铝 alumina ,aluminum oxide 氢氧化铝 Hydrogen alumina 电解铝electrolyse aluminum 镀铝aluminum plating 铝板Aluminum Board/plate/sheet 花纹铝板 Aluminum embossed sheet 彩铝板 colored Aluminum plate 纯铝板pure Aluminum plate 复合铝板clad aluminium 硬铝板 duralumin sheet 铝合金Aluminum alloy 锻用铝合金aluminum alloy for temper 铝铜合金aluminum copper alloy 铝基合金aluminum base alloy 铝铁合金aluminum iron alloy 铝镁合金aluminum magnesium alloy 铝镍合金aluminum nickel alloy 铝硅合金aluminum silicon alloy 铝基硅镁合金anticorodal 耐蚀铝合金corrosion-proof/ corrosion- resistant/ corrosion resisting aluminum alloy 铝黄铜aluminum brass 铝青铜aluminum bronze 高铝砖alumina brick 铝合金型材aluminium alloy sections 铝合金线Aluminum alloy wire 铝型材Aluminum profile 铝棒 Aluminum stick/rod 铝圆棒Aluminum round bar 铝箔 Aluminum foil 铝卷 Aluminum roll
氧化铝介质参数
Phone [301] 695-9400 ? Fax [301] 695-7065 ? trans .tech @https://www.wendangku.net/doc/8714588620.html, ? www. trans https://www.wendangku.net/doc/8714588620.html, 202896A ? Trans -Tech Propri etary I nformation ? Products and Product Information are Subject to Change Without Notice ? June 13, 2013 1 DATA SHEET Dielectric and Alumina Supports Dielectric Supports Dielectric supports can be used with all disc or cylinder type Dielectric Resonators (DRs) to improve coupling and temperature stability. Contact Trans -Tech , Inc. (TTI) for other support configurations . Figure 1. Disk and Cylinder Types Note: For disk type support, only use the D s and L s dimensions. s s Alumina Supports High Frequency Applications For high frequency applications (above 6 GHz), TTI offer s a special grade of alumina with the properties listed in Table 3. Table 3. Material Characteristics—Alumina Supports for High Cellular and PCS Applications For cellular and PCS frequencies , TTI offer s a different grade of alumina with the properties listed in Table 3. Note: Contact TTI’s factory for available sizes. Table 4. Material Characteristics—Alumina Supports for Cellular
氧化铝柱净化和石油废物分离 气相色谱法US EPA 3611B-1996
METHOD 3611B ALUMINA COLUMN CLEANUP AND SEPARATION OF PETROLEUM WASTES 1.0SCOPE AND APPLICATION 1.1Alumina is a highly porous and granular form of aluminum oxide. It is available in three pH ranges (basic, neutral, and acidic) for use in chromatographic cleanup procedures. Method 3611 utilizes neutral pH alumina to separate petroleum wastes into aliphatic, aromatic, and polar fractions. 1.2Method 3611 was formerly Method 3570 in the Second Edition of this manual. 1.3This method is restricted to use by or under the supervision of trained analysts. Each analyst must demonstrate the ability to generate acceptable results with this method. 2.0SUMMARY OF METHOD 2.1The column is packed with the required amount of adsorbent, topped with a water adsorbent, and then loaded with the sample to be analyzed. Elution of the analytes is effected with a suitable solvent(s), leaving the interfering compounds on the column. The eluate is then concentrated (if necessary). 3.0INTERFERENCES 3.1 A reagent blank should be performed for the compounds of interest prior to the use of this method. The level of interferences must be below the method detection limit before this method is performed on actual samples. 3.2More extensive procedures than those outlined in this method may be necessary for reagent purification. 3.3Caution must be taken to prevent overloading of the chromatographic column. As the column loading for any of these types of wastes approaches 0.300 g of extractable organics, separation recoveries will suffer. If overloading is suspected, an aliquot of the base-neutral extract prior to cleanup may be weighed and then evaporated to dryness. A gravimetric determination on the aliquot will indicate the weight of extractable organics in the sample. 3.4Mixtures of petroleum wastes containing predominantly polar solvents, i.e., chlorinated solvents or oxygenated solvents, are not appropriate for this method. 4.0APPARATUS AND MATERIALS 4.1Chromatography column: 300 mm x 10 mm ID, with Pyrex? glass wool at bottom and a polytetrafluoroethylene (PTFE) stopcock. NOTE:Fritted glass discs are difficult to decontaminate after highly contaminated extracts have been passed through. Columns without frits may be purchased. Use a small pad of Pyrex? glass wool to retain the adsorbent. Prewash the CD-ROM3611B - 1Revision 2 December 1996
耐火材料词汇
耐火材料词汇 A acid refractory 酸性耐火材料 addition 外加剂 adhesives 粘结剂 affinage furnace 精炼炉 agglutinant 烧结剂 agitator 搅拌机 air set binder 气硬性结合剂 air stove 热风炉 albite 钠长石 alcohol 乙醇 alpha-alumina a氧化铝 alpha phase a相 alumdum powder 刚玉粉 alumdum product 电熔刚玉制品 alumina 氧化铝 sintered alumina 烧结氧化铝 alumina ball 氧化铝球 alumina brick 高铝砖 alumina carbon brick 铝碳砖 alumina carbon refractory 铝碳质耐火材料alumina cement 高铝水泥 alumina-chrome brick 铝铬砖 alumina coats 氧化铝涂层 alumina content 氧化铝含量 alumina fibres 氧化铝纤维 alumina firebrick 氧化铝耐火砖高铝砖alumina-graphite submerged nozzle 铝碳浸入式水口 alumina industry 氧化铝工业 alumina light-weight brick 氧化铝轻质砖alumina-magnesia brick 铝镁砖 alumina powder 氧化铝粉 alumina products 氧化铝制品 alumina refractory 氧化铝质耐火材料alumina refractory bubble products 氧化铝耐火空心球制品 alumina-rich brick 高铝砖 alumina scale 氧化铝结瘤 alumina silica brick 铝硅砖 alumina silica refractory raw materials 硅酸铝质耐火原料 aluminate 铝酸盐 aluminate cement 铝酸盐水泥 aluminate trihydrate 氢氧化铝 alumina ware 氧化铝制品 aluminium 铝Al aluminium carbide 碳化铝 aluminium dust 铝粉 aluminium flakes 铝粉 aluminium hydrate 氢氧化铝 aluminium hydroxide 氢氧化铝aluminium nitride 氮化铝 aluminium orthophosphate 正磷酸铝aluminium oxide 氧化铝 aluminium oxide fiber 氧化铝纤维
氧化铝简介
名称 中文名称:铝氧,三氧化二铝 英文别名:Aluminum oxide 化学式 Al2O3 相对分子质量 101.96 性状 难溶于水的白色固体。无臭。无味。质极硬。易吸潮而不潮解。两性氧化物,能溶于无机酸和碱性溶液中,几乎不溶于水及非极性有机溶剂。相对密度(d204)4.0。熔点约2000℃。 储存 密封干燥保存。SCRC100009 用途 用作分析试剂。有机溶剂的脱水。吸附剂。有机反应催化剂。研磨剂。抛光剂。 质检信息质检项目指标值 水中溶解物,% ≤0.5 硅酸盐(SiO3) 合格 碱金属及碱土金属,% ≤0.50 重金属(以Pb计),% ≤0.005 氯化物(Cl),% ≤0.01 硫酸盐(SO4),% ≤0.05 灼烧失量,% ≤5.0 铁(Fe),% ≤0.01
物理性质 式量101.96 amu 熔点2303 K 沸点3250 K 真密度 3.97 g/cm3 松装密度:0.85g/mL(325目~0)0.9g/mL(120目~325目) 晶体结构三方晶系(hex) 导电性常温状态下不导电 热化学属性 ΔfH0liquid ?1620.57 kJ/mol ΔfH0solid ?1675.69 kJ/mol S0liquid, 1 bar 67.24 J/mol·K S0solid 50.9 J/mol·K 安全性 食入低危险 吸入可能造成刺激或肺部伤害 皮肤低危险 眼睛低危险 在没有特别注明的情况下,使用SI单位和标准气温和气压。 氧化铝是铝和氧的化合物,分子式为Al2O3。在矿业、制陶业和材料科学上又被称为矾土。 应急处理 隔离泄漏污染区,限制出入。建议应急处理人员戴防尘面具(全面罩),穿防毒服。避免扬尘,小心扫起,置于袋中转移至安全场所。若大量泄漏,用塑料布、帆布覆盖。收集回收或运至废物处理场所处置。
铝材专业词汇--中英文对照
铝型材行业词汇-铝材英语 铝锭Aluminum Ingot 氧化铝alumina ,aluminum oxide 氢氧化铝Hydrogen alumina 电解铝electrolyse aluminum 镀铝aluminum plating 铝板Aluminum Board/plate/sheet 花纹铝板Aluminum embossed sheet 彩铝板colored Aluminum plate 纯铝板pure Aluminum plate 复合铝板clad aluminium 硬铝板duralumin sheet 铝合金Aluminum alloy 锻用铝合金aluminum alloy for temper 铝铜合金aluminum copper alloy 铝基合金aluminum base alloy 铝铁合金aluminum iron alloy 铝镁合金aluminum magnesium alloy 铝镍合金aluminum nickel alloy 铝硅合金aluminum silicon alloy 铝基硅镁合金anticorodal 耐蚀铝合金corrosion-proof/ corrosion- resistant/ corrosion resisting aluminum alloy 铝黄铜aluminum brass 铝青铜aluminum bronze 高铝砖alumina brick 铝合金型材aluminium alloy sections 铝合金线Aluminum alloy wire 铝型材Aluminumprofile 铝棒Aluminum stick/rod 铝圆棒Aluminum round bar 铝箔Aluminum foil 铝卷Aluminum roll 铝带/铝条Aluminum strip 铝线Aluminum thread/wire 钢芯铝绞线aluminium cable steel reinforcing 铝管Aluminum pipe/tube 精拉铝管finishing Aluminum tube 伸缩铝管extension Aluminum tube 挤压铝管extruded aluminum pipe 铝管避雷器aluminium cell lightning arrester
铝合金的英语词汇
铝合金的英语词汇 产品Products 铝锭Aluminum Ingot 氧化铝alumina ,aluminum oxide 氢氧化铝Hydrogen alumina 电解铝electrolyse aluminum 镀铝aluminum plating 铝板Aluminum Board/plate/sheet 花纹铝板Aluminum embossed sheet 彩铝板colored Aluminum plate 纯铝板pure Aluminum plate 复合铝板clad aluminium 硬铝板duralumin sheet 铝合金Aluminum alloy 锻用铝合金aluminum alloy for temper 铝铜合金aluminum copper alloy 铝基合金aluminum base alloy 铝铁合金aluminum iron alloy 铝镁合金aluminum magnesium alloy 铝镍合金aluminum nickel alloy 铝硅合金aluminum silicon alloy 铝基硅镁合金,安奇可罗达耐蚀铝硅镁合金; 铝基硅镁合金anticorodal 耐蚀铝合金corrosion-proof/ corrosion- resistant/ corrosion resisting aluminum alloy 铝黄铜aluminum brass 铝青铜aluminum bronze 高铝砖alumina brick 铝合金型材aluminium alloy sections 铝合金线Aluminum alloy wire 铝型材Aluminum profile 铝棒Aluminum stick/rod 铝圆棒Aluminum round bar 铝箔Aluminum foil 铝卷Aluminum roll 铝带/铝条Aluminum strip 铝线Aluminum thread/wire 钢芯铝绞线aluminium cable steel reinforcing 铝管Aluminum pipe/tube 精拉铝管finishing Aluminum tube 伸缩铝管extension Aluminum tube 挤压铝管extruded aluminum pipe 铝管避雷器aluminium cell lightning arrester
奥图泰简介(英文)
Outotec in brief
A global leader in the development of minerals processing and metallurgi-cal technologies, Outotec has a distinguished history of achievement going back more than a hundred years. Tracing its roots to Outokumpu and Lurgi Metallurgie, Outotec has a long tradition of developing metallurgical pro-cesses which are both ef? cient and environmentally sustainable. Today, it has a wide range of customers within the iron and steel, aluminum and non-ferrous metals industries as well as in industrial minerals and other process industries. A global leader in clean technology Outotec experts design and deliver plants, processes and equipment worldwide, and provide engineering, project and support services on six continents. As a result of being environmentally sound and energy-ef? cient, dozens of Outotec technologies have become industry standards. This dedication to creating cleaner and more effective solutions has resulted in a number of technologies, which have been rated by the Euro-pean Union as the greenest economically viable option available. At the forefront of innovation To maintain its position at the forefront of innovation, Outotec has two in-house research centers, eight laboratories and four test plants. In addi-tion to this, the company has a global network of sales and service cen-ters. In 2007, Outotec’s over 2,000 professionals working together with a broad network of international subcontractors generated annual sales of EUR 1 billion. “We work in close partnership with our customers to identify the most pro? table solution for their business and help them stay ahead of the competition – to get more out of ore,” Tapani J?rvinen, President and CEO of Outotec, says. More out of ore! Outotec’s philosophy is embodied in the slogan “More out of ore”. It is the promise made to Outotec stakeholders - customers, shareholders and employees alike. As well as being a platform from which to communicate Outotec’s goals, it helps the company differentiate itself from its compe-titors. Put simply, Outotec has more technologies, products and services, more experience and expertise. And just as importantly, Outotec emplo- yees give more in everything they do.
耐火材料中英文词汇对照
acid refractory 酸性耐火材料 addition 外加剂 adhesives 粘结剂 affinage furnace 精炼炉 agglutinant 烧结剂 agitator 搅拌机 air set binder 气硬性结合剂 air stove 热风炉 albite 钠长石 alcohol 乙醇 alpha-alumina a氧化铝 alpha phase a相 alumdum powder 刚玉粉 alumdum product 电熔刚玉制品 alumina 氧化铝 sintered alumina 烧结氧化铝 alumina ball 氧化铝球 alumina brick 高铝砖 alumina carbon brick 铝碳砖 alumina carbon refractory 铝碳质耐火材料 alumina cement 高铝水泥 alumina-chrome brick 铝铬砖 alumina coats 氧化铝涂层 alumina content 氧化铝含量 alumina fibres 氧化铝纤维 alumina firebrick 氧化铝耐火砖高铝砖 alumina-graphite submerged nozzle 铝碳浸入式水口alumina industry 氧化铝工业 alumina light-weight brick 氧化铝轻质砖 alumina-magnesia brick 铝镁砖 alumina powder 氧化铝粉 alumina products 氧化铝制品 alumina refractory 氧化铝质耐火材料 alumina refractory bubble products 氧化铝耐火空心球制品alumina-rich brick 高铝砖 alumina scale 氧化铝结瘤 alumina silica brick 铝硅砖 alumina silica refractory raw materials 硅酸铝质耐火原料aluminate 铝酸盐 aluminate cement 铝酸盐水泥 aluminate trihydrate 氢氧化铝 alumina ware 氧化铝制品 aluminium 铝Al aluminium carbide 碳化铝
Aluminum
Aluminum Warren Haupin Consultant,Lower Burrell,Pennsylvania I.History of Aluminum II.Natural Occurrence of Aluminum Compounds III.Mining of Bauxite IV.Extraction of Pure Alumina V.Electrolytic Reduction of Alumina to Aluminum (Hall–H′eroult Process) VI.Energy Considerations VII.Alternative Processes for Producing Aluminum VIII.Production of Ultra-High-Purity Aluminum IX.Properties of Aluminum X.Alloys of Aluminum XI.Forming and Shaping Operations XII.Finishes for Aluminum https://www.wendangku.net/doc/8714588620.html,es of Aluminum XIV.Environmental Considerations GLOSSARY Anode effect Condition in which the electrolyte no longer wets the anode,creating a high electrical resistance. Bauxite Rock or earthy soil that contains a high concen-tration of aluminum hydroxide minerals. Bayer liquor Recycled aqueous solution of sodium hy-droxide(NaOH)and sodium aluminate(NaAlO2). Bayer process Process to extract pure alumina(Al2O3) from bauxite. Cold work Shaping metal by pressure at room tempera-ture. Desilication Removal of soluble silicates from Bayer liquor by forming insoluble sodium aluminum silicates called DSP(desilication product). Dewet To change a wetted surface to a nonwetted surface. Digestion High-temperature dissolution or extraction. Dolime Calcined dolomite rock(CaO+MgO). Grains(in metal)Microscopically resolvable crystals. Guinier-Preston(GP)zones Clusters of solute atoms in a supersaturated solid-solution lattice. Hall–H′e roult process Process for electrolytic reduction of alumina dissolved in molten cryolite. Ingot Cast block of metal suitable for mechanical shaping by rolling,forging,extruding,and similar processes or for remelting. 495
氧化铝简介
氧化铝 氧化铝,又称三氧化二铝,分子量102,通常称为“铝氧”,是一种白色无定形粉状物,俗称矾土。 郑州玉发集团是中国最大的白刚玉生产商。专注白刚玉和煅烧α氧化铝近30年,技术先进,品类齐全。联系QQ 2596686490,电话156390七七八八一。 目录 概要 物理性质 安全性 应急处理 中国工业概况 建议 用途 氧化铝制备及应用: 概要 物理性质 安全性 应急处理 中国工业概况 建议 用途 氧化铝制备及应用: ?什么是氧化铝 ?β型氧化铝 ?氧化铝企业发展策略 展开 概要 管制信息 本品不受管制
分子结构图 名称 中文名称:铝氧,三氧化二铝 英文别名:Aluminum oxide 化学式 Al2O3 相对分子质量 101.96 性状 难溶于水的白色固体。无臭。无味。质极硬。易吸潮而不潮解。两性氧化物,能溶于无机酸和碱性溶液中,几乎不溶于水及非极性有机溶剂。相对密度(d204)4.0。熔点约2000℃。 储存 密封干燥保存。SCRC100009 用途 用作分析试剂。有机溶剂的脱水。吸附剂。有机反应催化剂。研磨剂。抛光剂。 质检信息质检项目指标值 水中溶解物,% ≤0.5 硅酸盐(SiO3) 合格 碱金属及碱土金属,% ≤0.50 重金属(以Pb计),% ≤0.005 氯化物(Cl),% ≤0.01 硫酸盐(SO4),% ≤0.05
灼烧失量,% ≤5.0 铁(Fe),% ≤0.01 物理性质 式量101.96 amu 熔点2303 K 沸点3250 K 真密度 3.97 g/cm3 松装密度:0.85g/mL(325目~0)0.9g/mL(120目~325目) 晶体结构三方晶系(hex) 导电性常温状态下不导电 热化学属性 ΔfH0liquid ?1620.57 kJ/mol ΔfH0solid ?1675.69 kJ/mo l S0liquid, 1 bar 67.24 J/mol·K S0solid 50.9 J/mol·K 安全性 食入低危险 吸入可能造成刺激或肺部伤害 皮肤低危险 眼睛低危险 在没有特别注明的情况下,使用SI单位和标准气温和气压。 氧化铝是铝和氧的化合物,分子式为Al2O3。在矿业、制陶业和材料科学上又被称为矾土。 应急处理 隔离泄漏污染区,限制出入。建议应急处理人员戴防尘面具(全面罩),穿防毒服。避免扬尘,小心扫起,置于袋中转移至安全场所。若大量泄漏,用塑料布、帆布覆盖。收集回收或运至废物处理场所处置。
